'Product Blog'
The Use of Cp2Ni as a Precursor in ALD/CVD Processes
Potential Issues May be Encountered Using Nickelocene (28-1301) as an ALD/CVD Precursor
Nickel and Nickel oxide have been widely used in the electronics industry. Nickelocene or Bis(cyclopentadienyl)nickel (28-1301), has been used as a precursor to form nickel oxide and elemental nickel films by CVD and ALD.1,2 For Nickel metal films, the oxide is firstly formed using oxidizing agents (e.g. H2O, ozone or di-oxygen) and then reduced using hydrogen at a deposition temperature of 165⁰C. 3,4 Nickel has also been used to form thin films of Nickel Silicide (NiSi), as well as for rechargeable batteries and ferromagnetic random access memories (RAMs). Cp2Ni has been used as a Nickel source in CVD for making NiFe2O4 (NFO) magnetostrictive thin films.5
Atomic Layer Deposition (ALD) is used to deposit smooth, homogeneous thin films with good conformality. Precise thickness control is allowed from self-limiting sequential reactions. One very important property required in an ALD precursor is its thermal stability. Precursors must be sufficiently volatile at a temperature at which they do not decompose thermally. Very often the thermal profile of a potential precursor is determined using thermogravimetric analysis (TGA).2 For the TGA of Cp2Ni, it appears that there is a clean sublimation from 170⁰C to 190⁰C, leaving ca. 5% residue after decomposition at 186⁰C.
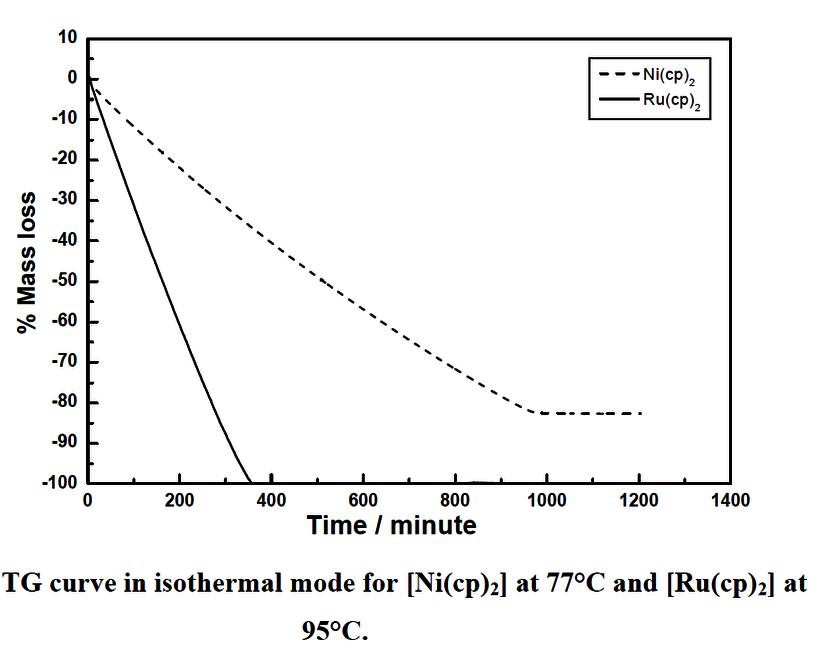
More recently it has come to our attention that the apparent stability of Cp2Ni as profiled by the TGA may not present the whole story. TGA analysis shows thermal stability from a relatively short thermal timeframe. Kang and Rhee2 report that no appreciable decomposition of Cp2Ni occurs at 120⁰C by holding Cp2Ni isothermally in a sublimator and examining its gas phase IR spectra using a heated gas cell. Various temperature gas phase spectra were taken. However, a time factor was not taken into account. Decomposition is also a time dependent process and therefore one should wait isothermally for some longer period of time after which one can confirm whether prolonged heating (thermal stress) causes further decomposition or not.6 With this in mind, a further study6 shows that Cp2Ni decomposition can be detected at a low temperature of 77⁰C, and that after a prolonged period of time (15 h at 77⁰C), the mass loss becomes constant leaving 18% residual mass (see Fig 1). With shorter timeframes (e.g. 2 h) one could infer pure evaporation and the decomposition is not as obvious, and in fact may not be evident at all.
Figure 1
In agreement with this data is the investigation of returned cylinders of Nickelocene which were used for ALD purposes. Complaints were generally regarding issues with blockages occurring with loss of ability to flow out of the cylinder, or there being an unexpected early loss of precursor flux from the cylinder. Analysis of the cylinders showed the presence of decomposed material in the cylinders, as well as in the narrower inlet and more predominantly outlet lines and valves of the cylinders. This can be clearly seen in the following pictures:

Solids at VCR connection between cylinder and valve (cylinder side)

Solids at top of cylinder near valve assembly (viewed via sliced end of cylinder)

Decomposed (brown) material coating fill port of cylinder

Outlet valve blockage
Conclusion:
We can offer the following general recommendations:
1.) When determining whether a precursor is a suitable candidate for ALD/CVD use, one should consider carrying out a more prolonged “thermal stress test” as well as the routine shorter term thermal analysis techniques more commonly employed. This more rigorous testing will enable the researcher to gain a more complete picture of the thermal behavior of a precursor and may affect the ultimate selection of a potential precursor for use.
2.) When using Nickelocene, if it is possible to use the cylinder at lower temperatures (say 70⁰C or less) then decomposition may be lessened.
3.) Similarily, decomposition may be lessened and the useful life of the precursor lengthened if heating is suspended during any "non-run time" (down time between runs).
4.) Where possible, consider using an alternative material such as Strem 28-0045 for example which is thermally more stable.
References:
1. Electrochem. Solid-State Lett. 2002, vol. 5, issue 6, C64-C66
2. J. Mater. Res., 2000, vol. 15, No. 8.
3. Chemical vapor deposition, 2011, 17, 177-180
4. Thin solid films, 2001, 391, 57-61.
5. ECS Journal of Solid State Science and Technology, 2014, 3 (11), P345-P352.
6. Experimental investigations of thermodynamic properties of organometallic compounds. Thesis; R.A. Siddiqui, section 4.1.3. University of Duisburg-Essen, 2009.
Products mentioned in this blog and related products:
28-1301: Bis(cyclopentadienyl)nickel, 99% (Nickelocene), CAS # 1271-28-9
28-0083: Bis(ethylcyclopentadienyl)nickel, min. 98%, CAS # 31886-51-8
28-0085: Bis(pentamethylcyclopentadienyl)nickel, 98% (Decamethylnickelocene),CAS # 74507-63-4
28-0086: Bis(i-propylcyclopentadienyl)nickel, min. 98%, CAS # 57197-55-4
28-0045: Bis(N,N'-di-t-butylacetamidinato)nickel(II), (99.999%-Ni) PURATREM, CAS # 940895-79-4