'Product Blog'
CALLERY™ (-)/(+)-Diisopinocampheylchloroboranes — Chiral Boranes for Total Synthesis
(+)-DPC and (-)-DPC selective reducing agents play important role in the synthesis of complex natural products
Chlorodiisopinocampheylboranes ((+)-DPC and (-)-DPC, sometimes abbreviated as (+)-/(−)-Ipc2BCl or (+)-/(−)-DIP-Cl), are excellent enatio- and stereoselective reducing agents for a variety of functional groups providing enantiomerically enriched products. High yields, low impurities and simple isolation of the resultant products make chiral boranes the preferred reagents for several reduction reactions. These include asymmetric hydroboration, chiral reduction, asymmetric allyl-,crotylboration and, homologation, chiral enolboration and enantioselective ring-opening reactions. [1].
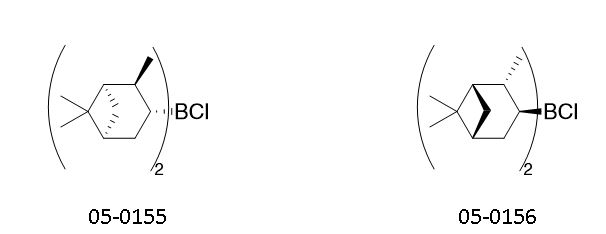
Table. 1. Chiral reducing agents CALLERY™ (-)-Diisopinocampheylchloroborane, ((-)-DPC (Catalog # 05-0155) and CALLERY™ (+)-Diisopinocampheylchloroborane, ((+)-DPC (Catalog # 05-0156) are available as 60-65% solution in hexanes or heptanes
Often, (+)-DPC and (-)-DPC boron chlorides are converted to the corresponding chiral B-allyldiisopinocampheylborane by the reaction of a desired allylmagnesium or allyllithium reagent with chlorodiisopinocampheylboranes. These B-allyldiisopinocampheylboranes has become important reagents especially in the synthesis of complex natural products for the formation of numerous secondary chiral centers [2-8]. An interesting direct one pot synthesis of B-allyl and B-allenyldiisopinocampheylborane reagents using allyl or propargyl halides and indium metal has been recently reported [9].
The intrinsic reduction ability of (+)-DPC and (-)-DPC to convert aldehydes and ketones to chiral alcohols are widely used in the total synthesis of natural products such as, Bruceollines J [10] and Bruceollines I [11] and Inthomycins A, B, and C [12].
References:
- Organoboranes for Syntheses; Amer. Chem. Soc.: Washington, DC, 2001; Chapter 1, pp 1-15.
- J. Org. Chem. 2005, 70, 8932.
- Org. Lett. 2008, 10, 261.
- Synlett 2008, 4, 569.
- Angew. Chem., Int. Ed. 2008, 47, 1733.
- Angew. Chem., Int. Ed. 2008, 47, 3242.
- J. Org. Chem. 2010, 75, 4095.
- ACS Omega 2020, 5, 18472.
- J. Org. Chem. 2012, 77, 4342.
- Org. Lett. 2013, 15, 4485.
- J. Nat. Prod. 2017, 80, 2384.
- J. Org. Chem. 2020, 85, 4795.
Featured Products
05-0155 CALLERY™ (-)-Diisopinocampheylchloroborane, 60-65% solution in heptanes ((-)-DPC) (85116-37-6)
05-0156 CALLERY™ (+)-Diisopinocampheylchloroborane, 60-65% solution in hexanes ((+)-DPC) (112246-73-8)
Related Products