'Product Blog'
Ghaffar-Parkins Catalyst for the Hydration of C≡N Bonds
The outstanding catalytic activity and selectivity of the platinum complex provide an effective hydration of nitriles
In 1995, Ghaffar and Parkins [1] introduced the hydride-platinum(II) complex [PtH{(PMe2O)2H}(PMe2OH)] (fig. 1), a commercially available catalyst for the hydration of nitriles (C≡N bonds). The alternative acid/base transformation mechanism of nitriles into amides usually requires strict conditions, mainly due to yield and selectivity issues, since these classical methods are often unable to control the over-hydrolysis of the primary amide product [2, 3].
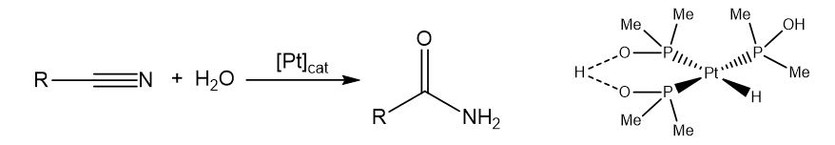
Figure 1. Reaction for the catalytic hydration of nitriles and
the structure of the Ghaffar-Parkins catalyst
The Ghaffar-Parkins catalyst demonstrates outstanding activity and selectivity for the conversion of a large number of natural products and biologically active molecules [3]. It can operate under relatively mild conditions and is characterized with exceptionally high functional group compatibility. This mild hydration catalyst converts hindered and acid/base sensitive nitriles to amides. The amide is released from the metal coordination sphere preventing further hydrolysis to acid. Typically, turnover frequency (TOF) ranges from 16 to 380 h-1 depending on steric factors. For optimal results the presence of coordinating anions especially cyanide and halide should be avoided. However, catalytic hydration of nitriles is limited in α-hydroxynitriles, where cyano and a hydroxy group are attached to the same carbon atom. The hydration rates for these types of substrates are very low. [4, 5]
The platinum complex has become an irreplaceable catalyst especially for the total synthesis of a large number of natural products, biologically active molecules, and fine chemicals in the pharmaceutical industry [3]. Some synthetic processes involving the catalyst are also summarized in the corresponding technical note.
References:
- Tetrahedron Lett. 1995, 36, 8657.
- Comprehensive Organic Functional Group Transformations, 2005, Volume 5, 201-294.
- Appl. Sci. 2015, 5, 380.
- Inorg. Chem. 2009, 48, 7828.
- J. Inorg. Organomet. Polym. 2014, 24, 145.
Featured Product:
78-0725 Hydrido(dimethylphosphinous acid-kP)[hydrogen bis(dimethylphosphinito-kP)]platinum(II) Ghaffar-Parkins catalyst (173416-05-2)
Related Products & Resources:
Catalysts
Metal Catalysts for Organic Synthesis