'Product Blog'
Potassium Graphite Electrodes for Potassium Ion Batteries
Electrochemically intercalated potassium graphitic materials bring potassium ion batteries to a new level
A decade ago, the market for lithium-ion batteries (LIBs) was primarily regulated by their utilization in portable electronics. However, because of the increasing popularity of electric vehicles, the total demand for lithium is forecast to triple by 2025, and prices for lithium carbonate (93-0312; 93-0311; 03-0800) and hydroxide (93-0329), used in LIBs will continuously increase. Parallel increases raise concern regarding the shortage and geographical abundance of Li resources. This fact led to a rise in research attention to alternative technologies [1-2], such as sodium-ion (SIBs) and potassium-ion batteries (KIBs).
In our previous blog, we report briefly about the Graphite Intercalation Compounds (GICs) that are made by intercalation of guest species (alkali metals) between the graphite layers. By adapting the type and concentration of guest elements, GICs are able to possess tunable electrical properties.
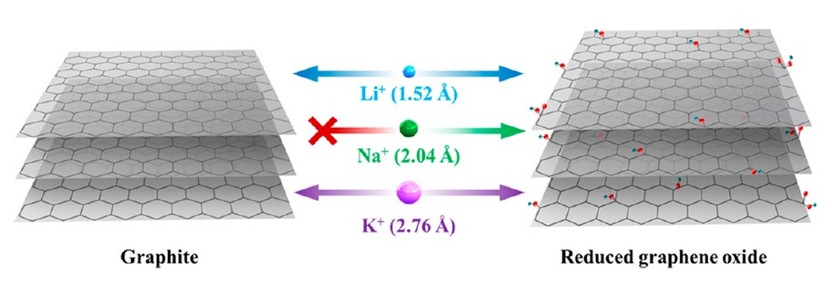
Figure 1. Schematic illustration of electrochemical intercalation of Li, Na, and K ions into graphite and reduced graphene oxide (RGO). Li and K ions can electrochemically intercalate into both graphite and RGO, while Na ions can only be electrochemically intercalated into RGO.
Reprinted with permission from Nano Lett. 15, 11, 7671-7677.
Copyright 2015 American Chemical Society
Graphite is a common anode material in batteries and has a moderate Li storage capacity (∼350-370 mAh/g) at approximately 0.1 V vs. Li/Li+[3]. Studies on SIBs demonstrated that graphite does not properly intercalate sodium ions [4-6]. The limited capacity is mainly attributed to the larger size of Na ions compared to the interlayer spacing in graphite, as well as a weak interaction of intercalated Na species with carbon layers (Fig. 1) [7]. With potassium atoms being even heavier than lithium or sodium, one may expect that potassium ions are more difficult to intercalate between the graphite layers. However, because of its high natural abundance and low cost, potassium is highly accepted in K ion-based energy storage research.
There are very few types of KIBs known and they’re based on O2/K [8], S/K [9], and Prussian blue [10, 11] systems. For battery applications, KC8 (19-1935), the stage-one K-GIC has been synthesized by potassium vapor transport or by soaking graphite in a nonaqueous solution with potassium metal solvated [12]. Nevertheless, real progress for K-containing cathode/graphite batteries similar to conventional LIBs has been achieved by electrochemical intercalation of K into the graphite [3, 13-14], that results in formation of stage-1 KC8 compound (Fig. 2) delivering 273 mAh/g [3], 244mAh/g [13] and 207 mAh/g [14] of reversible capacity respectively. These recent findings open new opportunities for the future of K-ion based battery technologies.
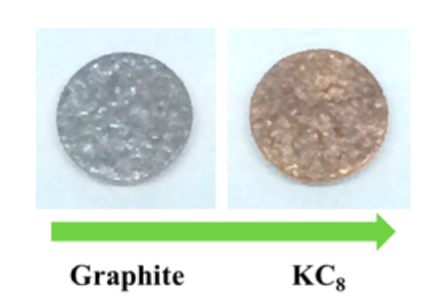
Figure 2. Image of graphite electrode and electochemicaly intercaleted KC8
Reprinted with permission from Nano Lett. 15, 11, 7671-7677.
Copyright 2015 American Chemical Society
For more information about the products for next generation lithium ion batteries, please review our booklet on energy related materials and blog on silicon based electrolytes.
References:
- ACS Appl. Mater. Interfaces 2017, 9, 4404.
- Chem. Soc. Rev., 2017, 46, 3529.
- J. Am. Chem. Soc., 2015, 137, 11566.
- Energy Environ. Sci., 2013, 6, 2338.
- J. Electrochem. Soc,. 2001, 148, A803.
- J. Nano Lett., 2012, 12, 3783.
- Nat. Nanotechnol., 2015, 10, 980.
- J. Am. Chem. Soc., 2013, 135, 2923.
- J. Inorg. Chem., 2014, 53, 9000.
- Nano Lett., 2011, 11, 5421.
- Nat. Commun., 2012, 3, 1149.
- J. Am. Chem. Soc., 2015, 137, 11566.
- Electrochem. Commun., 2015, 60, 172.
- Nano Lett., 2015, 15, 7671.
Products Mentioned in Blog:
93-0312: Lithium carbonate (99.99%-Li) PURATREM (554-13-2)
93-0311: Lithium carbonate, 99% (554-13-2)
03-0800: Lithium carbonate (99.999%-Li) PURATREM (554-13-2)
93-0329: Lithium hydroxide monohydrate, min. 98% (1310-66-3)
19-1935: Potassium graphite KC8 (12081-88-8)
Related Resources:
Materials for Energy Applications Booklet
Materials for Battery Applications: Redox Shuttles & Electrolyte Solvents
Graphene Products
Carbon-Based Nanomaterials & Elemental Forms Booklet