'Product Blog'
Potassium Graphite KC8 – An Excellent Reducing Agent
Electronic Properties of Potassium Intercalated Graphite Promotes a Variety of Uses in Chemical Synthesis

This deep, golden-bronze solid of potassium intercalated graphite KC8 (19-1935) is quite a spectacular material. Its impressive color is attributed to its unique structure comprising potassium atoms localized between planar graphite layers (Fig. 1). In 1965, G.R. Hennig recognized that the color of the intercalated compound indicates the extent of charge transfer from the potassium atoms to the graphite.1-2 As a result, this brilliantly colored material exhibits a range of electronic and redox properties for potential applications.
KC8 is a fundamental example and frequently studied material within the unique class of Graphite Intercalation Compounds (GICs) which feature graphite based host-guest systems. The host (graphite) interacts with the guest (potassium) via charge transfer.
Plain graphite has only delocalized electrons due to the sp2 hybridized carbon atoms. In comparison, KC8 has a higher electrical conductivity thanks to its rapid electron exchange. Therefore, additional delocalized electrons wander across the graphite layers while electrons available for donation are accessible on the surface. For additional review, references 3 & 4 contain a more sophisticated description of the electronic structure of KC8.
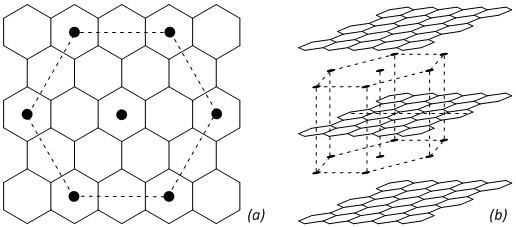
Fig. 1. Simplified structure of potassium intercalated graphite KC8:
(a) 2D projection and (b) 3D side view
Potassium graphite is an extremely strong reducing agent and has been used extensively as a catalyst in chemical synthesis. Powdered KC8 does not dissolve in organic solvents and acts in a heterogeneous manner, exploiting its high surface area. A portion of the potassium atoms located at the intercalated edges are easier to access for the reaction. These atoms have a reduction potential similar to that of metallic potassium. The KC8 behavior is in accordance with the chemistry of free potassium. Many papers cite the successful use of KC8 for synthetic properties. The strong reducing ability of KC8 can be utilized for purposes such as the formation of multiple bonds between elements, polymerization catalysis, coupling reactions in organic synthesis, and the construction of various organometallic complexes.5-11
References:
- Synthetic Metals, 1995, 74, 201.
- J. Chem. Phys., 1965, 43, 1201.
- Phys. Stat. Sol., 1996, 196, 131.
- Phys. Rev. B., 2009, 79, 205106.
- Organometallics, 2007, 26, 4737.
- J. Am. Chem. Soc.,2007, 129, 12412.
- J. Am. Chem. Soc., 2014, 136, 5460.
- ACS Cent. Sci., 2017, 3, 217.
- Angew. Chem. Int. Ed., 2018, 57, 1874.
- Inorg. Chem., 2018, 57, 20.
- Chem. Eur. J., 2018, 24, 826.
Products mentioned in this blog and related products:
19-1935: Potassium graphite KC8 [12081-88-8]
19-1990: Potassium (99.95%) (breakseal ampoule) [7440-09-7]
19-1910: Potassium sodium alloy 78:22 (99.95%) [11135-81-2]
93-1990: Potassium (99.95%) (prescored ampoule) [7440-09-7]
Additional literature available:
Materials for Battery Applications Redox Shuttles & Electrolyte Solvents